Helpful! Why Is Buffer Important In Biological Systems
Buffers help to maintain a normal pH of the biological systems. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system.
The higher the buffer concentration the greater the pH control.
Why is buffer important in biological systems. Four important generalizations about buffers. Their optimal buffering capacity or range is defined by the dissociation constant or ka of the acid. How do buffers affect pH.
By definition a buffer system is a solution that resists a. What do buffers do and why are they important in biological systems. Explain how a buffer system works.
Small molecules such as bicarbonate and phosphate provide buffering capacity as do other substances such. Our stomachs secrete hydrochloric acid. From the lab what does buffer capaciry depend on.
We commonly discuss buffering capacity in terms of the pKa or the logarithmic constant of ka. A buffer solution consists of two components. Many biochemical reactions involved in vital processes like metabolism respiration the transmission of nerve impulses and muscle contraction and relaxation take place within a.
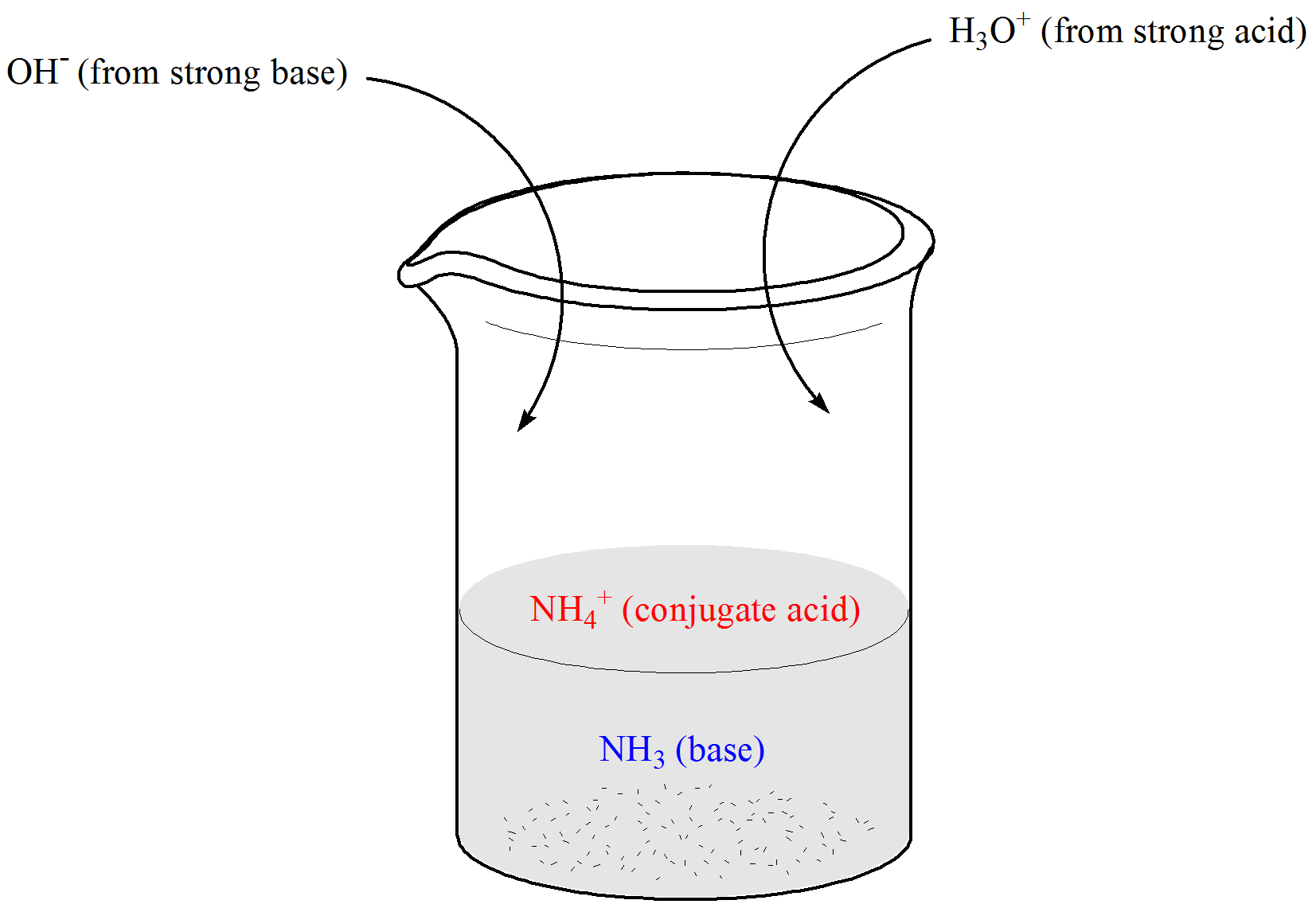
You can illustrate your point using e addition of a base or an acid. In other words a buffer is an aqueous solution of either a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer solutions are extremely important in biology and medicine because most biological reactions and enzymes need very specific pH ranges in order to work properly.
No matter what the buffer concentration maximum pH control is reached when HA A-. Why are biological buffers important. Maintenance of biological pH is important because cells only function over a narrow pH change.
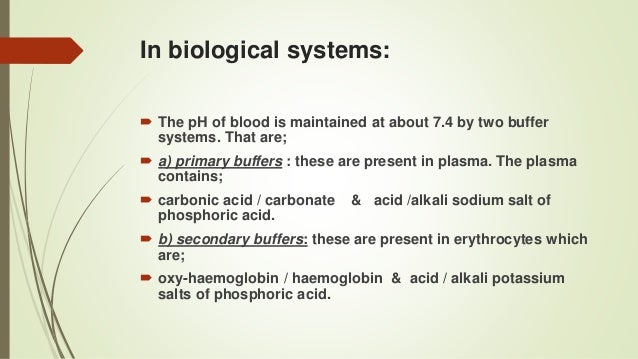
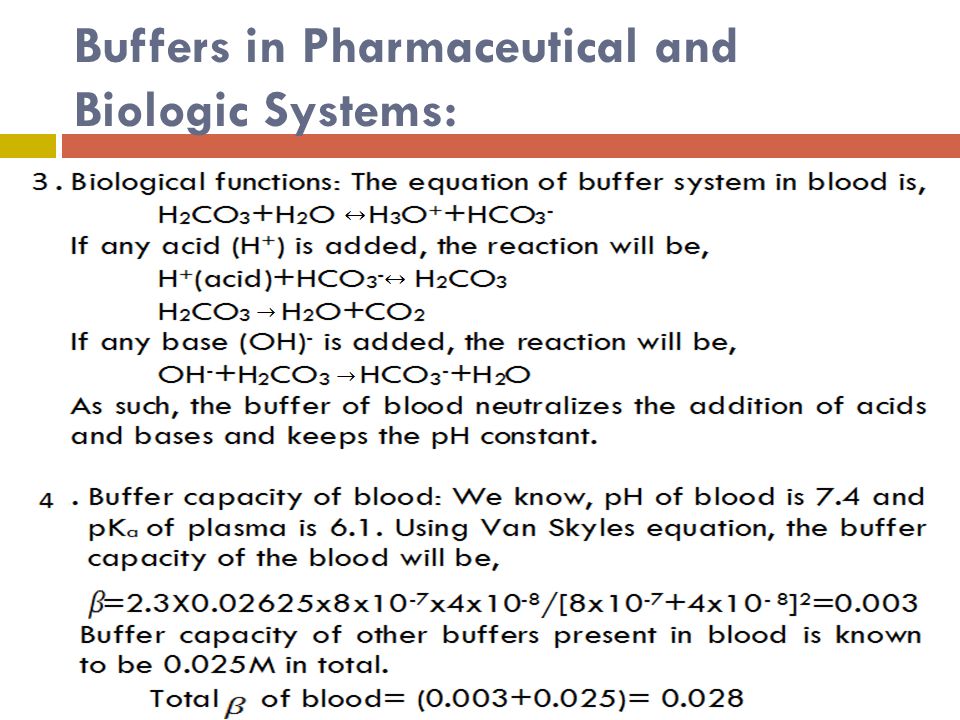
Buffers are an important part of the biochemical processes of living things because they help keep the pH within organisms body stable. Cellular respiration produces carbon dioxide as a waste product. The bodys chemical buffer system consists of three individual buffers out of which the carbonic acid - bicarbonate buffer is considered most important.
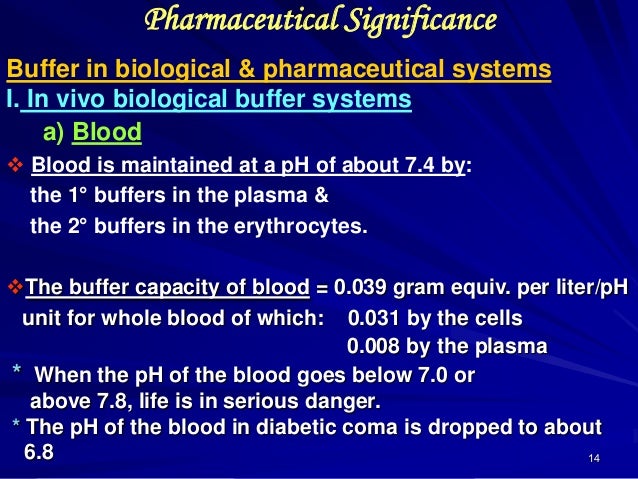
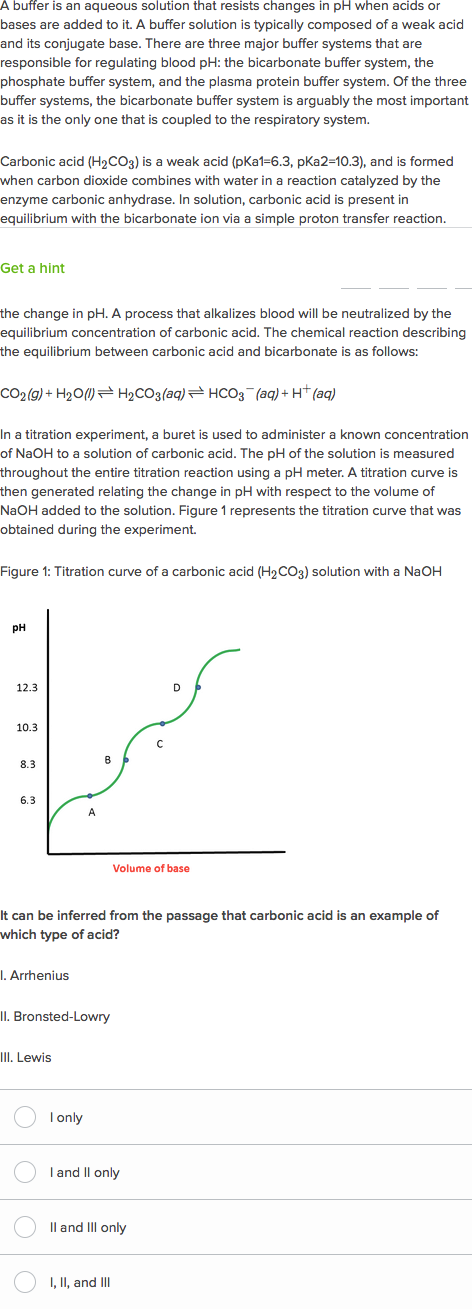
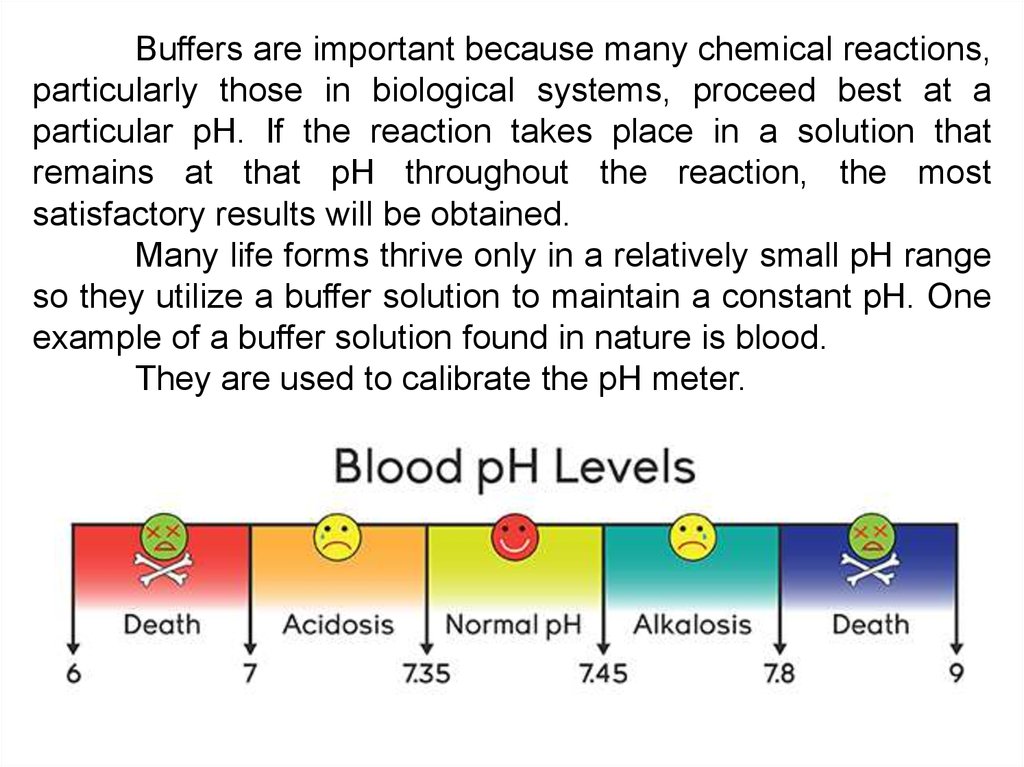
Blood Human blood contains a buffer of carbonic acid H 2 CO 3 and bicarbonate anion HCO 3 - in order to maintain blood pH between 735 and 745 as a value higher than 78 or lower than 68. Buffers are the mixture of weak acids and their salts of strong bases or the mixture of weak bases and their salts of strong acids. This is important for processes andor reactions which require specific and stable pH ranges.
Buffers are important in biological systems because of their ability to maintain constant pH conditions. For example a buffer with a pH of 68 has a pH buffering range of 58-78. Both the rates of biochemical reactions and their equilibrium constants are very sensitive to the availability of hydronium ions.
The higher the buffer capacity of the treated water the more efficient its purification with a hydrolyzing coagulant. Most biochemical reactions that are essential for life only take place in a narrow pH range. This is hydrolysed into bicarbonate ion in the blood.
It is useful in the biological system because proteins and enzymes are highly susceptible to degradation and denaturation at any time if there is a change in pH. A buffer system is a solution that prevents the drastic change in the concentration of H ions and pH in the system. Buffers are able to find any extra H or OH- ions and prevent pH change.
Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. A buffer may also be called a pH buffer hydrogen ion buffer or buffer solution. The optimal pH level of the blood is 74 which is maintained by three different types of buffer systems working in the body 2The addition of an acid or a base to a substance changes its pH level.
While in the blood this bicarbonate ion serves to neutralise acids introduced in to the blood through. Buffering is important in living systems as a means of maintaining a fairly constant internal environment also known as homeostasis. It is able to neutralize small amounts of added acid or base thus maintaining the pH of the solution relatively stable.
Buffers are important in biological systems because of their ability to. In nature they offer protection to living organisms while in labs theyre used to create an environment with a stable pH. A buffer is a solution that resists changes in pH when.
A buffer is a chemical substance that helps maintain a relatively constant pH in a solution even in the face of addition of acids or bases. Buffering is important in living systems as a means of maintaining a fairly constant internal environment also known as homeostasis. Most buffers consist of a weak acid and a weak base.
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. Biological buffers are compounds that help the body maintain a pH around 74. Weak base and its conjugate acid.
The presence of buffers ensures that the bodys pH remains in this range despite changes in the surroundings. A buffer is composed of an equilibrium mixture of a weak acid HA and its conjugate weak base A-. Buffers are of great importance in living systems.
Weak acids and bases do not completely dissociate in water and instead exist in solution as an equilibrium of dissociated. Buffer systems play important roles in nature and in laboratory settings. Buffers are used to maintain a stable pH in a solution as they can neutralize small quantities of additional acid of base.
A flowchart may be useful. View the full answer. Here we examine the basic chemistry of buffer systems and how that chemistry applies to reactions in experimental biological systems.
The pH or the amount of hydrogen ions H in a solution level of the blood is important in ensuring the proper functionality of biological systems 2. Buffers consist of a weak acid HA and its conjugate base A or a weak base and its conjugate acid. Buffers play an important role in the chemical treatment of water to separate it from suspended matter of coagulation.
Based on the addition of NaoH to water describe why buffers are important in biological systems 3. What is importance of buffer solution in biological process. When an acid or alkali has added the pH of the solution changes in the absence of buffers.
We consider the buffering capacity of a specific buffer to be the pKa 1.

Buffers Definition Overview Expii
Buffer And Isotonic Solution Ppt Video Online Download

Chemical Buffer Systems And Acid Base Balance
The Role Of The Bicarbonate Buffer System In Regulating Blood Ph Practice Khan Academy
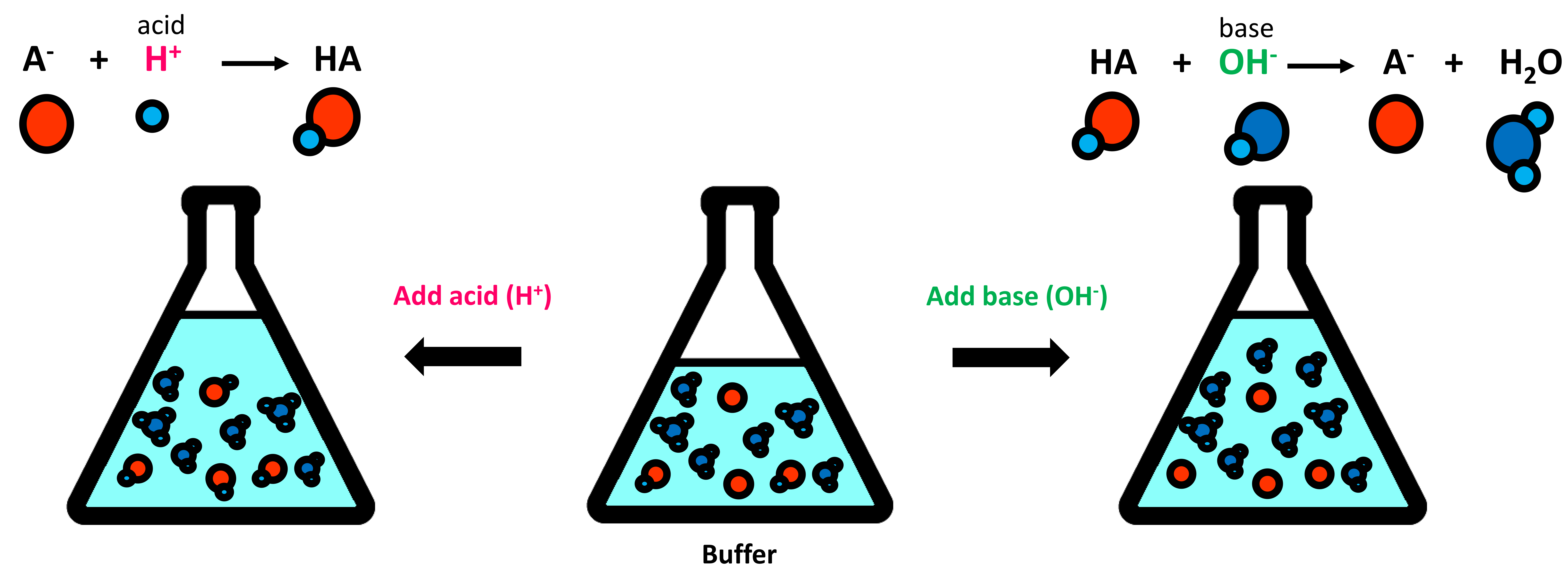
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy

Buffers What Are The Importance Of Buffers In Biological System
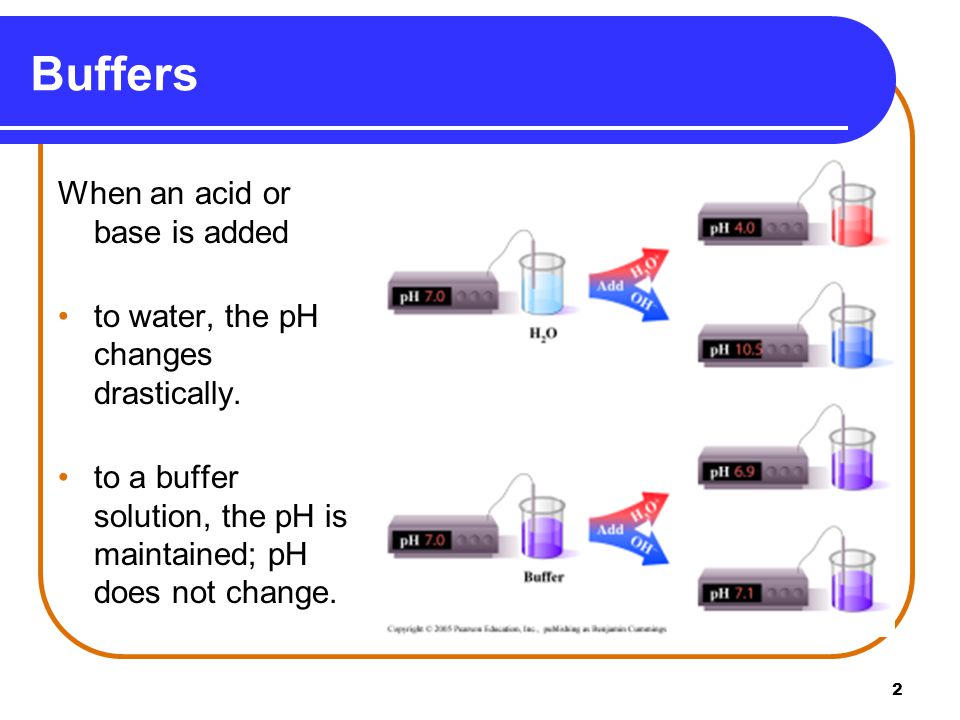
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes

Introduction To Buffers Video Khan Academy

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
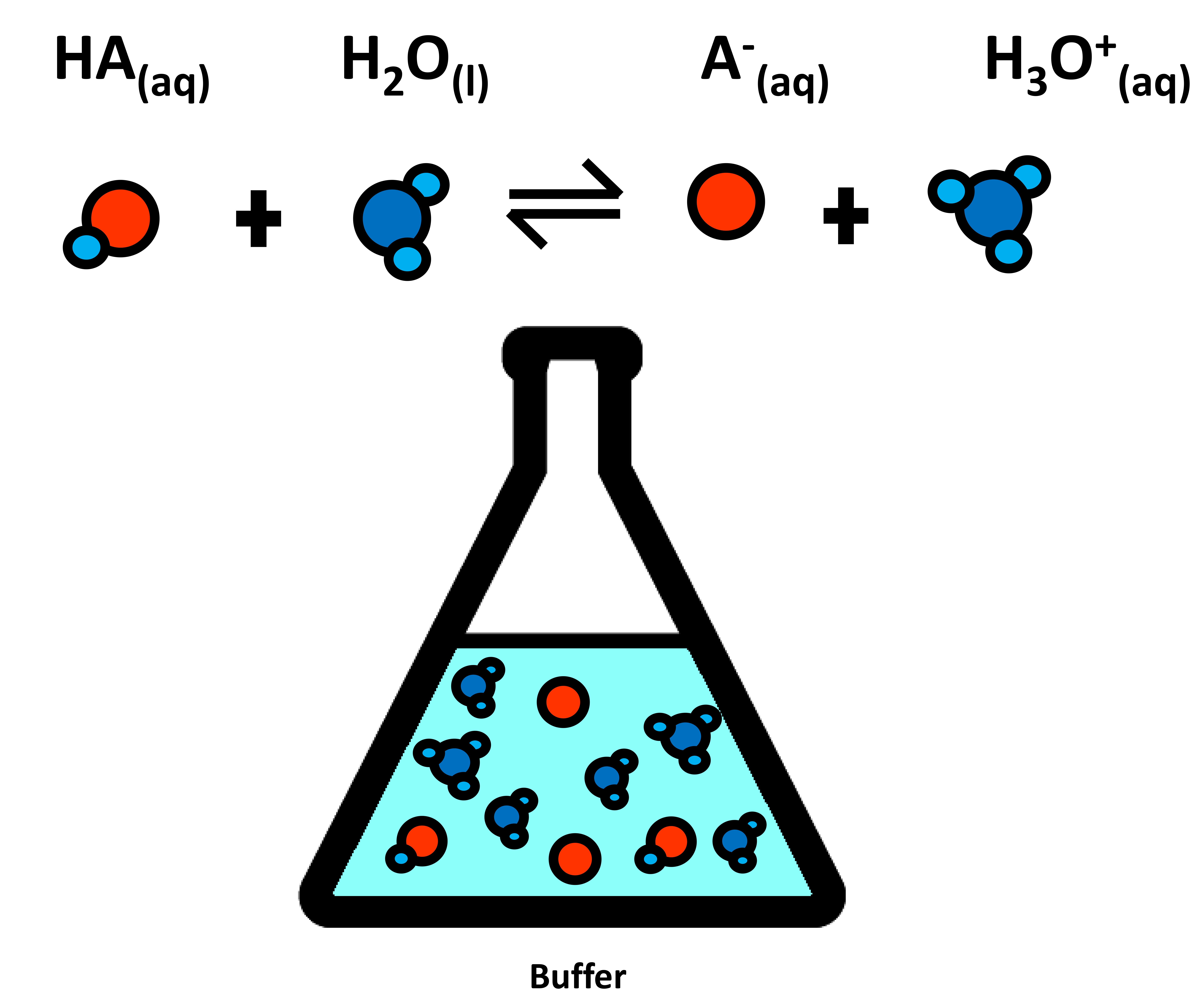
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
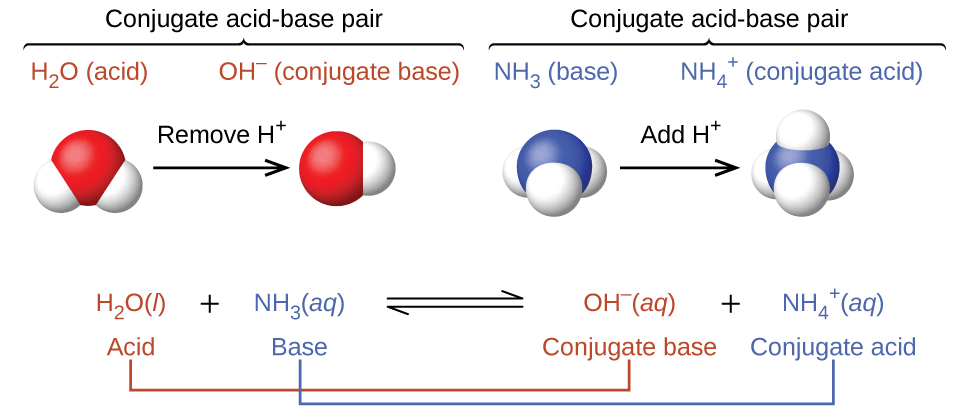
Buffers Definition Overview Expii

Buffers What Are The Importance Of Buffers In Biological System

Biological Buffer Systems Youtube

Buffers Definition Overview Expii
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Autoionization Of Water Hydrolysis Of Salts Prezentaciya Onlajn