Incredible! Why Don T Hydrocarbons Dissolve In Water
This phenomenon is responsible for the like-dissolves-like statements that are often found in introductory chemistry books including this. Water is not miscible with diethyl ether.

Chemistry Ii Water And Organic Molecules
These molecules often have polar groups called esters groups of atoms that contain CO bonds at one end.

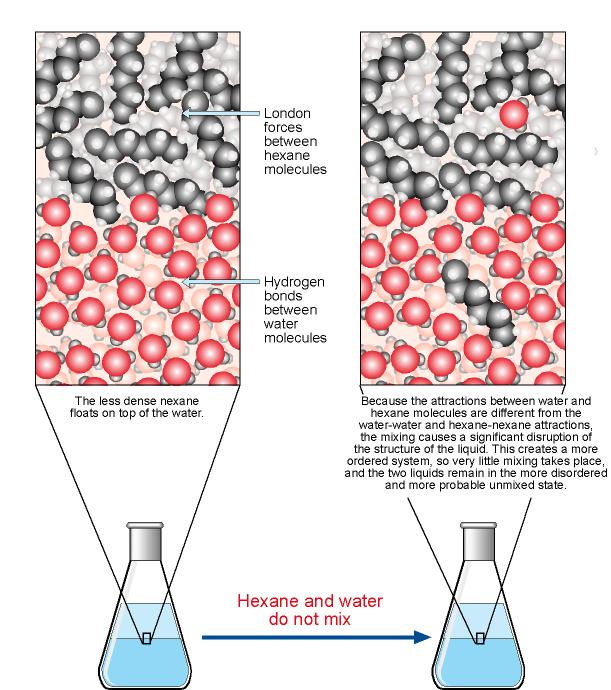
Why don t hydrocarbons dissolve in water. In confined environments eg. Would be good to show water molecules packed densely In one glass of water there are more molecules than the number of known stars in the universe. Since non-polar substances are held together by weak van der Waals interactions and water molecules use strong hydrogen bonds the van der Waal interactions of non-polar substances are not strong enough to break the hydrogen bonds of the water molecules.
When oil is spilt at sea the rate of weathering depends on the nature of the oil water temperature wave action and use of dispersants. The reason why it is not able to dissolve in water is because the energy released in the formation of interaction between water and Hydrocarbon is insufficient to compensate for the energy abosrb to break the Instantaneous dipole-induced Dipole interaction Id-Id Td-Id hence it. As a result we might expect carbon tetrachloride to be very soluble in water.
Alcohol molecules have only one polar area and also have a larger nonpolar area. Oils for want of a better word are oily - they are slippery and to risk being tedious do not mix with water. Oil is a generic name for a group of compounds many of which are hydrocarbons or contain hydrocarbon-like regions.
Because carbon tetrachloride is a nonpolar molecule the interactions between adjacent molecules are very weak. Possibly the best illustration of this is the alcohol series. Propanol and above have limited aqueous solubilities.
However water molecules form strong hydrogen bonds with one another causing them to stick tightly to one another. Removing one or more hydrogen atoms from the hydrocarbon so it forms a multiple bond or replacing. 114 Once you get more than six carbons in the chain these groups do not greatly influence solubility in water just as the single O H groups in most alcohols do not greatly influence solubility.
Because the interaction between oil and water molecules is insufficient to overcome the interaction between water and water and oil and oil molecules. Because the difference between the electronegativities of carbon and hydrogen is small EN 040 hydrocarbons are nonpolar. Why do Hydrocarbons not dissolve in water.
The general solubility rule is like dissolves like meaning polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes. Whereas oils long chain hydrocarbons are non-polar. The solubility of a solute in a specific solvent depends on the like dissolves like principle.
The smaller molecules tend to emulsify with the water and are more difficult to remove from industrial water prior to discharge. Microbes in the soils and water have a natural ability to breakdown many of these compounds and any hydrocarbon that is exposed to. Extending this concept the solubility of NaCl is much less when you move onto methanol because by introducing the methyl group you.
A general rule of which you have heard is that like dissolves like. The polar water dissolves the polar coloring and the polar sugar. Polar ammonia molecules dissolve in polar water molecules.
Hydrocarbons do not dissolve in water because they are nonpolar compounds and water is a polar solvent. When two compounds are mutually insoluble theyre immiscible with each other. This means that nonpolar.
Hydrocarbons are not soluble in water. Small freshwater streams or lakes biodegradation will result in reduction in dissolved oxygen while there can be a localised build-up of toxic fractions Green Trett 1989. These molecules mix readily because both types of molecules engage in hydrogen bonding.
The alcohol is 30 water and 70 alcohol and is not a good dissolver. So oily molecules are primarily non-polar and interact with one another as well as with other molecules. So when you mix hydrocarbons with water they tend to form layers.
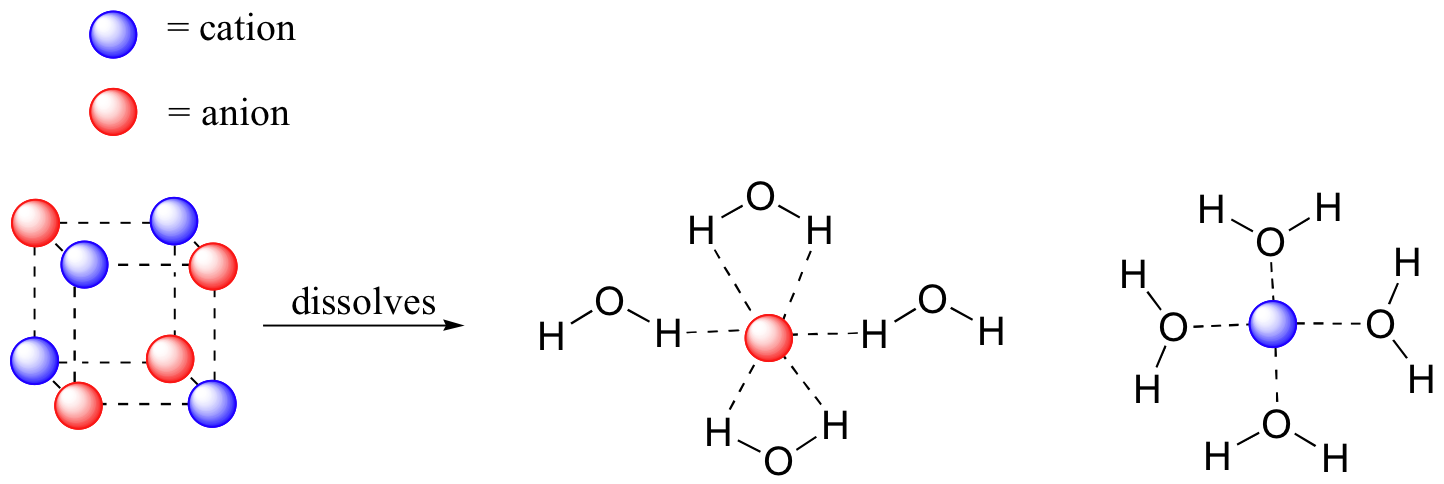
Since the intermolecular attractions are roughly equal the molecules can break away from each other and form new solute NH3 solvent H2O hydrogen bonds. Some PAHs are known carcinogens but many of these have not been measured in drinking-water. Once the salt dissolves in water the oil floats back up to the top of the water.
Water is more dense than more hydrocarbons so it is usually the bottom layer. Methanol and ethanol are infinitely miscible in water. The molecules of water are packed very densely.
What is the reason behind. As the length of the hydrocarbon chain increases the non-polar hydrocarbon part of the molecule starts to become more important and the solubility decreases. As a starting point you can think of water as the most polar alcohol its technically not an alcohol because it doesnt have any hydrocarbon functionalities attached - lots of salts are soluble in it as well as a lot of highly polar substances such as NaCl.
Oil molecules are not polar so they cannot dissolve either the coloring or the sugar. From the article it is clear that why oil does not mix with water and floats on it. The first reason that water and oil dont mix is because their molecules are packed differently.
The extreme case of such an environment which exhibits no force of attraction to the water molecules would be a. See full answer below. Water the PAHs detected in the highest concentrations are fluoranthene FA phenanthrene pyrene PY and anthracene.
When deciding solubility the guiding principle is that likes dissolve likes. If a solvent has polar character and the solute is nonpolar then solute cannot get dissolve in water. Long chain hydrocarbons have no electronegative functionality that.
Of the six PAHs usually measured in water for regulatory purposes FA is the only PAH that is detected to any significant extent. The family of compounds known as the hydrocarbons contain only carbon and hydrogen. Hydrocarbons lacking the stronger attractive forces present in water do not attract a water molecule as strongly as do the other water molecules in liquid water.
Water is strongly polar ie. Accordingly the non-polar substance cannot disperse itself in water. As a result they do not dissolve in polar solvents such as water.
Oil and Water have different densities. What is not quite so well known is Why. Because the C-C and C-H bonds that characterize hydrocarbon chains are relatively non-polar and are not able to participate in efficient hydrogen bonding.

Chemistry Ii Water And Organic Molecules

Chemistry Ii Water And Organic Molecules
Polar And Non Polar Solubility
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts

Penguinone Is An Organic Compound With The Molecular Formula C10h14o Its Name Comes From Th Cooking Steak On Grill How To Cook Steak Cooking With Coconut Oil

Lluciz Nasa New Horizons Nasa Missions Dwarf Planet

Chemical Reaction These Bubble Are Not Derived From The Physical Change When A Liquid Changes To A Gas But Inste Chemical Reactions Chemical Equation Bubbles

Overview Of Napl And Groundwater Programs In 2021 Groundwater Flow Map Floating In Water
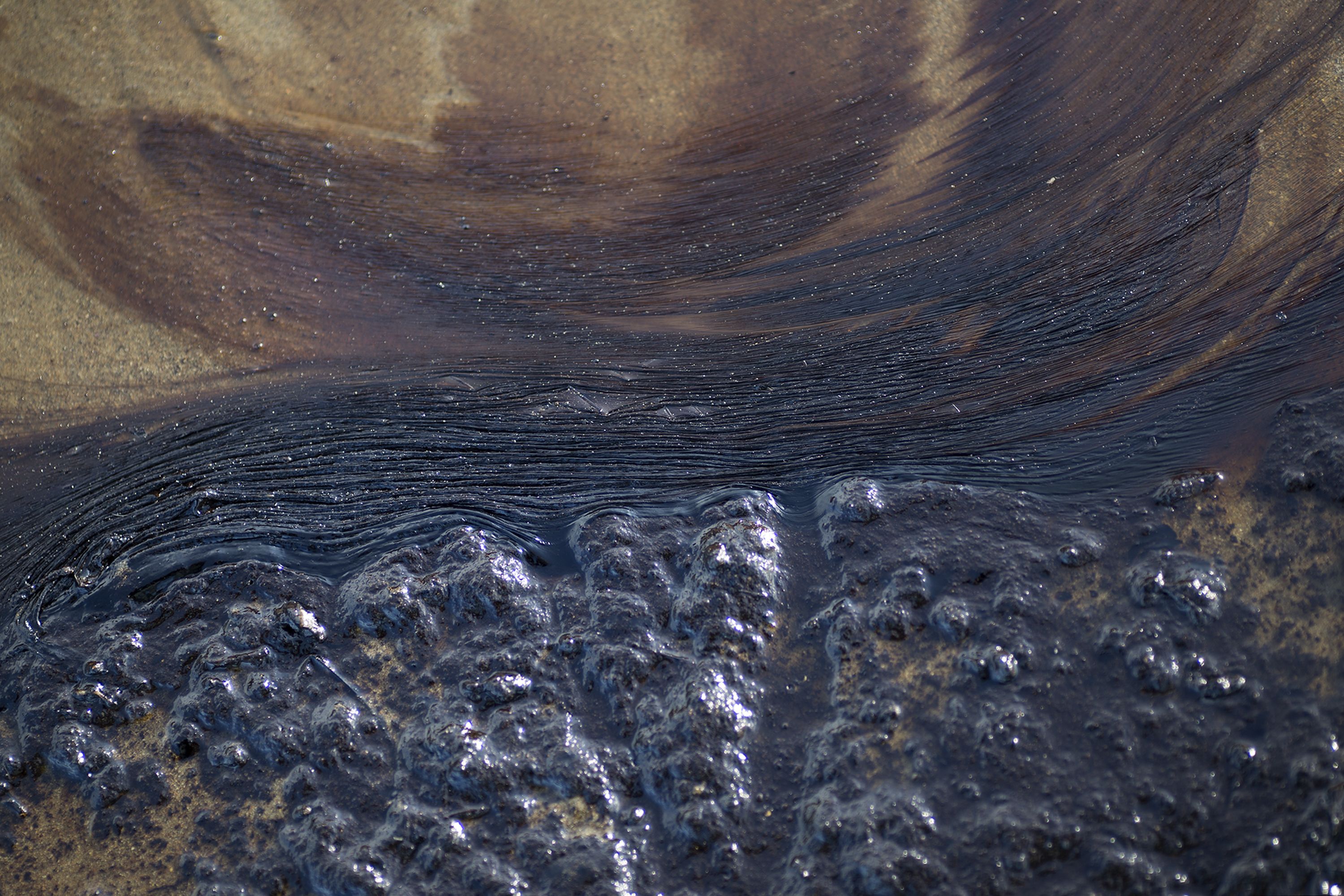
How Oil Breaks Down In Water Deepwater Cleanup Efforts

Pathophysiology Exam 2 Pg 2 Questions 5 9 Exam Study Materials Teaching

Benzene Is An Organic Compound That Is A Colorless Or Light Yellow Liquid That Has A Relatively High Melti Science Chemistry Study Chemistry Teaching Chemistry

Benzene Derivatives In Organic Chemistry Poster By Compound Interest Organic Chemistry Organic Chemistry Study Chemistry Help

Alcohols Phenols Ders Alcohols And Phenols May Be Viewed As Organic Derivatives Of Water Alcohols And Phenols Have A Com Functional Group Alcohol Solubility

A Ring Guard Protects Your Ring From Falling Off When It Is Too Loose Potential Threats To A Loose Ring Inclu How To Make Rings Make A Ring Smaller Ring Guard

Ingredients For Life Revealed In Meteorites That Fell To Earth Study Also Suggests Dwarf Planet In Asteroid Belt May Be A Source Of Rich Organic Matter Meteorite Asteroid Belt Salt Crystal

Chemistry Ii Water And Organic Molecules