WOW..Why Are Hydrogen Bonds Important In The Body
Functions of Hydrogen in the Human Body. Hydrogen bonding is responsible for waters unique solvent capabilities.
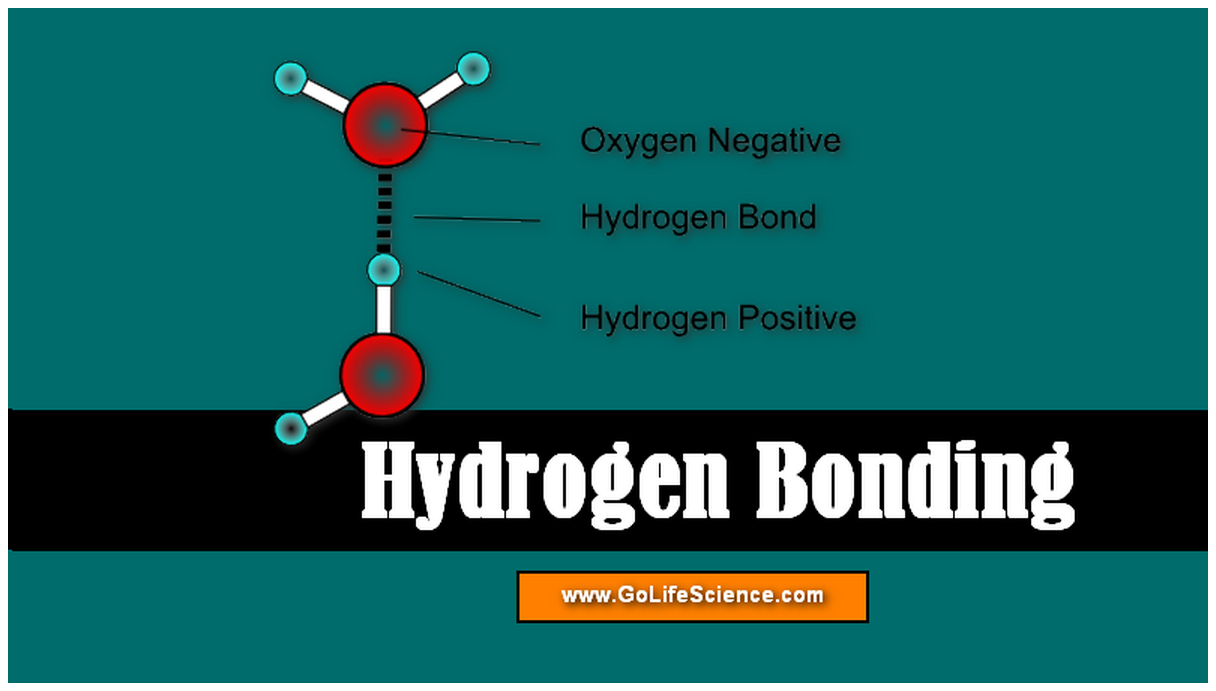
Hydrogen Bonding What Is Hydrogen Bonding And Its Types
Water is made up of hydrogen and oxygen and is absorbed by the cells of the body.

Why are hydrogen bonds important in the body. These are ionic bonds covalent bonds and hydrogen bonds. Hydrogen bonds are important in the body because many of the enzymes antibodies and other active protein molecules in various physiological processes. The proteins in the subunits are coiled into helices that are held together by hydrogen bonds.
Hydrogen bonding is responsible for waters unique solvent capabilities. Between the Functions of hydrogen in the human body The most important is to keep it hydrated. Three types of chemical bonds are important in human physiology because they hold together substances that are used by the body for critical aspects of homeostasis signaling and energy production to name just a few important processes.
Hydrogen bonds are a great example of why I love the chemistry part of biochemistry so much. Hydrogen bonding is important in many chemical processes. This is possible because water is composed of two hydrogen bonds and one of oxygen H2O that are responsible for being absorbed by the body cells.
Hydrogen helps in ATP to ADP develpoment which helps the body move. From the tiny world of the nucleus and the principles of intercellular forces the properties of. Therefore it is a crucial.
Can be stored in body such as ions of calcium phosphate in bones or be available individually for physiological processes. Hydrogen bonds are important in forming the secondary structures of proteins the helix and the pleated sheet. Hydrogen bonds hold complementary strands of DNA together and they are responsible for determining the three-dimensional structure of folded proteins including enzymes and antibodies.
A dipole is a molecule that is electrically neutral. Hydrogen Bonds Are Dipole-Dipole Attraction. It carries both a positive and negative charge with the different charges separated by only a small space.
Hydrogen bonds are important in forming the secondary structures of proteins the helix and the pleated sheet. And theyre important in the body because they caused D N A. When polar covalent bonds containing hydrogen form the hydrogen in that bond has a slightly positive charge because hydrogens electron is pulled more strongly toward the other element and away from the.
The most important function of hydrogen in the human body is to keep you hydrated. Bond is a weak bond between a hydrogen at Adam between hydrogen Adam and on oxygen or nitrogen Adam. Hydrogen bonds keep our DNA together.
What are hydrogen bonds and how are they important in the body. A hydrogen bond is a weak bond between a hydrogen atom and an atom of oxygen or nitrogen between molecules or different regions of a very large molecule. An atom that has either gained or lost electron s ion formed when they gainlose electrons to attain a full valence shell giving electrical charge.
Hydrogen bonds provide many of the critical life-sustaining properties of water and also stabilize the structures of proteins and DNA the building block of cells. They cause DNA to retain its double helix structure and contribute to the folding of proteins. Hydrogen is an element that is present in all the fluids of the human body allowing the.
Without the hydrogen bonds to keep its shape hemoglobin would be unable to function. Without the hydrogen bonds to keep its shape hemoglobin would be unable to function. We are asked to explain what hydrogen bonds are and how they are important in the body.
So howd you didnt. A hydrogen bond however is comparatively weak to covalent or ionic bond as much as 22 times time weaker Libes 2009 so in order to explain why hydrogen bonds are so necessary in life it is perhaps not significant that hydrogen bonds are weak on their own since the majority of their use within strong structures is facilitated by their strength as a. Hydrogen is very important to the human body.
Hydrogen bonding is a special type of chemical bond that involves dipole-dipole attraction between two or more dipolar molecules which are also referred to simply as dipoles. The hemoglobin molecule consists of four subunits. Hydrogen bonding is important in many chemical processes.
How are hydrogen bonds important in the body. Hydrogen bonding is responsible for waters unique solvent capabilities. Hydrogen bonds hold complementary strands of DNA together and they are responsible for determining the three-dimensional structure of folded proteins including enzymes and antibodies.
Hydrogen bonds hold complementary strands of DNA together and they are responsible for determining the three-dimensional structure of folded proteins including. Hydrogen bonds hold complementary strands of DNA together and they are responsible for determining the three-dimensional structure of folded proteins including enzymes and antibodies. See full answer below.
1 Hydrogen bonding in water. Why are hydrogen bonds important in the body. The proteins in the subunits are coiled into helices that are held together by hydrogen bonds.

Chemistry Tutorial Hydrogen Bond Water Molecule Chemistry

What S Up With That The Mysterious Effect That Makes Hot Water Freeze Faster Than Cold Hydrogen Bond Water Molecule Hydrogen Atom
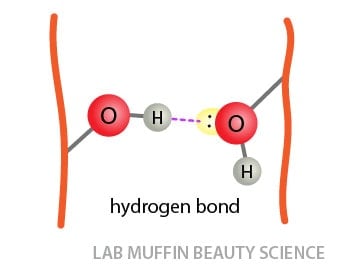
Hair Frizz Science Water And Hydrogen Bonds Lab Muffin Beauty Science
What Is The Energy Of A Hydrogen Bond

What Is Hydrogen Bonding Definition Examples Types Formation Applications Class 9 Class 11 Youtube

Homopolysaccharides Structures Macromolecules Biochemistry Chemical Structure

Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube
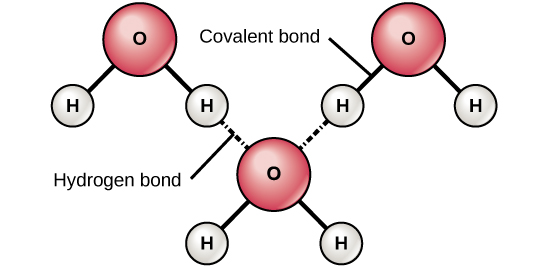
Why Life Depends On Water Biology For Non Majors I
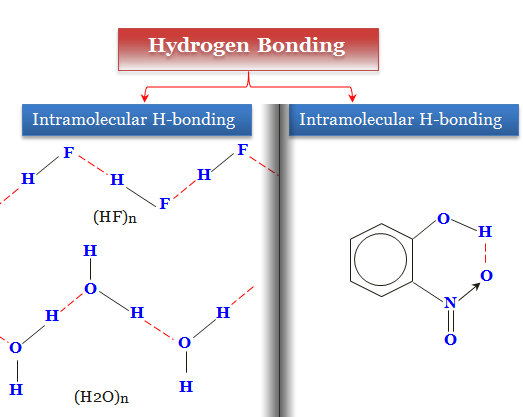
Hydrogen Bonding Definition And Examples In Chemistry By Chemistry Topics Medium

Water Has Both A Hydrogen Bond And A Polar Covalent Bond Hydrogen Bond Chemistry Classroom Covalent Bonding

Hydrogen Bonding Video Khan Academy

9 Hydrogen Bond Examples In Real Life Studiousguy
9 Hydrogen Bond Examples In Real Life Studiousguy
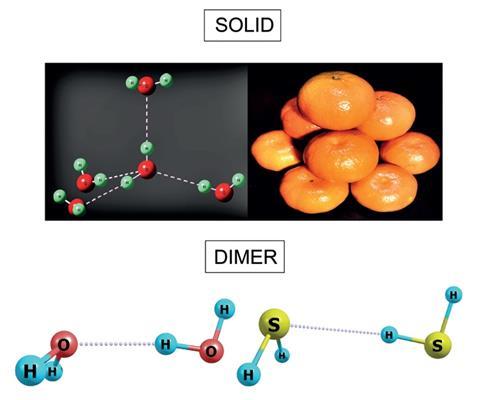
Hydrogen Sulfide Surprises As It S Discovered To Have Hydrogen Bonds Research Chemistry World

Chemistry Tutorial Hydrogen Bond Water Molecule Chemistry

Human Anatomy And Physiology Diagrams Hydrogen Bonds Hydrogen Bond Covalent Bonding Biochemistry

Hydrogen Bonds Make Water Sticky Manoa Hawaii Edu Exploringourfluidearth

Pattern Of Hydrogen Bonding In Water And Ice Hydrogen Bond Bond Organic Chemistry
